
Whereas the less number of protons than electrons makes the atom anionic. If the number of protons is more than electrons in an atom, it becomes cationic. Q. How do protons determine the charge of an atom?Īns. Charge of an atom completely depends on the number of protons in an atom. Unlike electrons, protons do not take part in a chemical reaction. The combined mass of proton and neutron virtually makes the mass of an atom. A strong nucleic force binds protons and neutrons together. Thus, in the periodic table, we arrange the elements according to their atomic number. Its absolute charge is 1.6022 x 10-19 coulomb of a positive charge.īesides, the atomic number is the number of protons present in one atom of that element. The absolute mass of a proton is 1.6726 x 10-27 kg. We consider an atom as neutral because it has the same number of protons and electrons. The positive charge of protons makes the nucleus also positive. Electrons are present outside the nucleus in a fixed orbit that we know as a shell. Besides, the protons and neutrons are present in a small nucleus at the center of the atom. After Neil Bohr’s atomic model it became easier to position the electrons, protons, and neutrons. This experiment ultimately led to the discovery of the atomic nucleus.
Rutherford to design the gold foil experiment. Interestingly, Thomson’s model later led E. Thomson’s model was rejected as it failed to determine the exact position of subatomic particles. Layer on Rutherford name these positively charged particles as ‘protons’. – The rays contain positively charged particles as they repel towards the cathode plate. – When the atmospheric pressure was gradually decreased below 1 atm a gas illumination was seen at the back of the cathode plate. – Later, A high voltage current of about 10kv was allowed to pass through the tube. – He makes the cathode plate porous and applies a layer of zinc sulphide on it. – He takes a gas-filled tube with positive and negative electrodes on two sides. Some important points of the experiment are as follows:. We also call these anode rays as canal rays. Protons were discovered by the anode ray experiment which was conducted by E. Anode Ray Experiment and Discovery of Protons We represent Electrons as ‘e−’, protons as ‘p+’, and neutrons as ‘n’. Goldstein discovered protons with the help of anode ray experiment. In 1932, James Chadwick discovered another subatomic particle, neutrons.
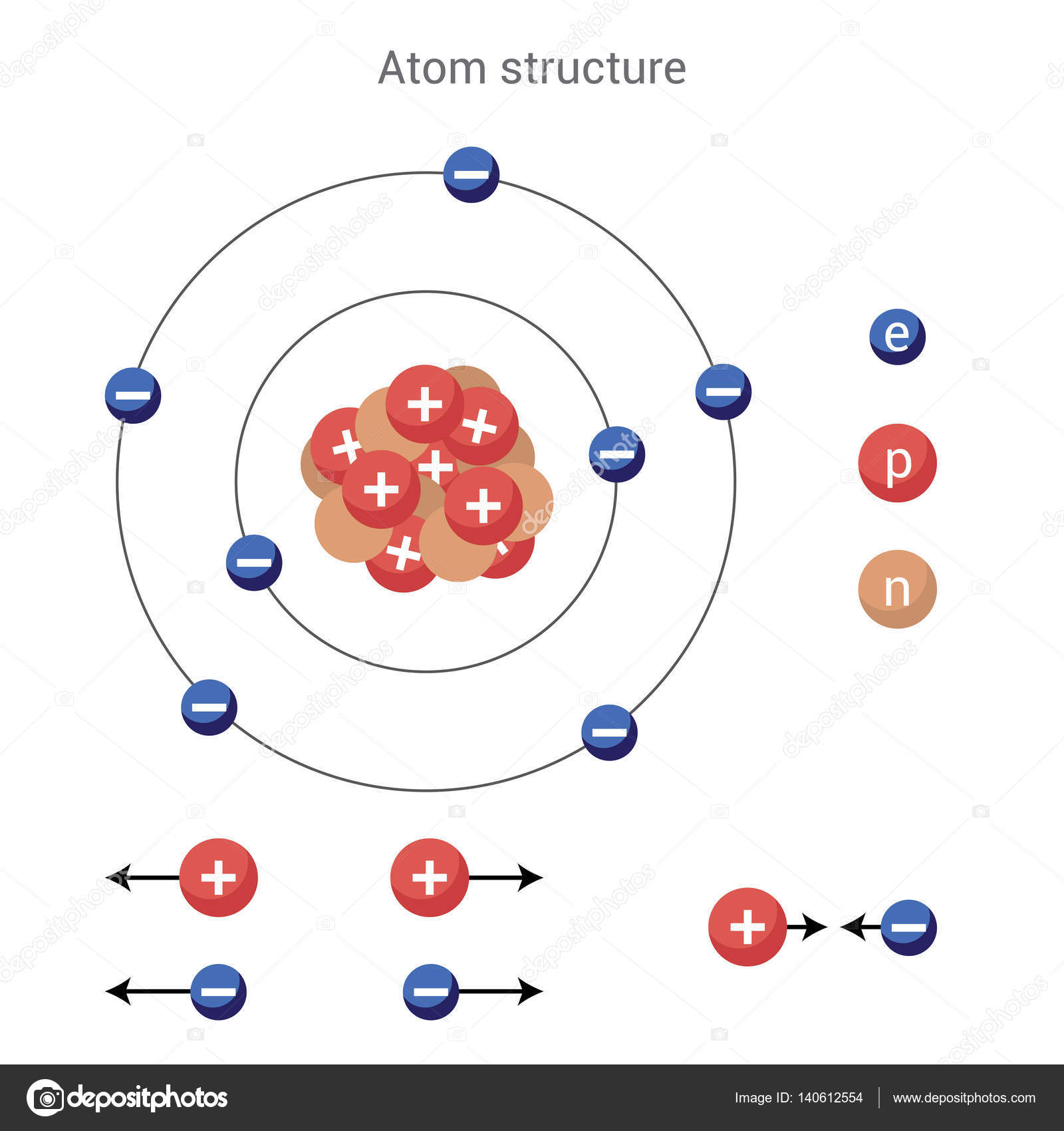
Thus, the cathode ray tube experiment of Thomson led to the discovery of electrons. The charge of proton is positive while electrons have a negatively charge whereas neutrons have no charge.Ī model for the structure of an atom was first proposed by J.

With further experiments, scientists came to know that atoms are further made up of three subatomic particles, electrons, protons and neutrons. Later on a Greek philosopher, Democritus suggests that on dividing a matter we can obtain an indivisible particle, which is indivisible.įurthermore, he pronounces it an atom. In this topic, we will teach you about mas and charge of a proton.Īround 500BC an Indian philosopher, Maharishi Kanad said that if we go on dividing the matter we shall get smaller particles. Charge of proton depends upon the number of electron and proton it contains.


 0 kommentar(er)
0 kommentar(er)
